Study, Lawsuits Reveal Alarming Relationship Between Talcum Powder and Cancer
Research and recent litigation against cosmetic manufacturer Johnson & Johnson have revealed that talcum powder products, including baby powder, put women's health at risk. Talc has been linked to an increased risk of ovarian cancer, and a study suggests that talcum powder may contain small particles of carcinogenic asbestos.
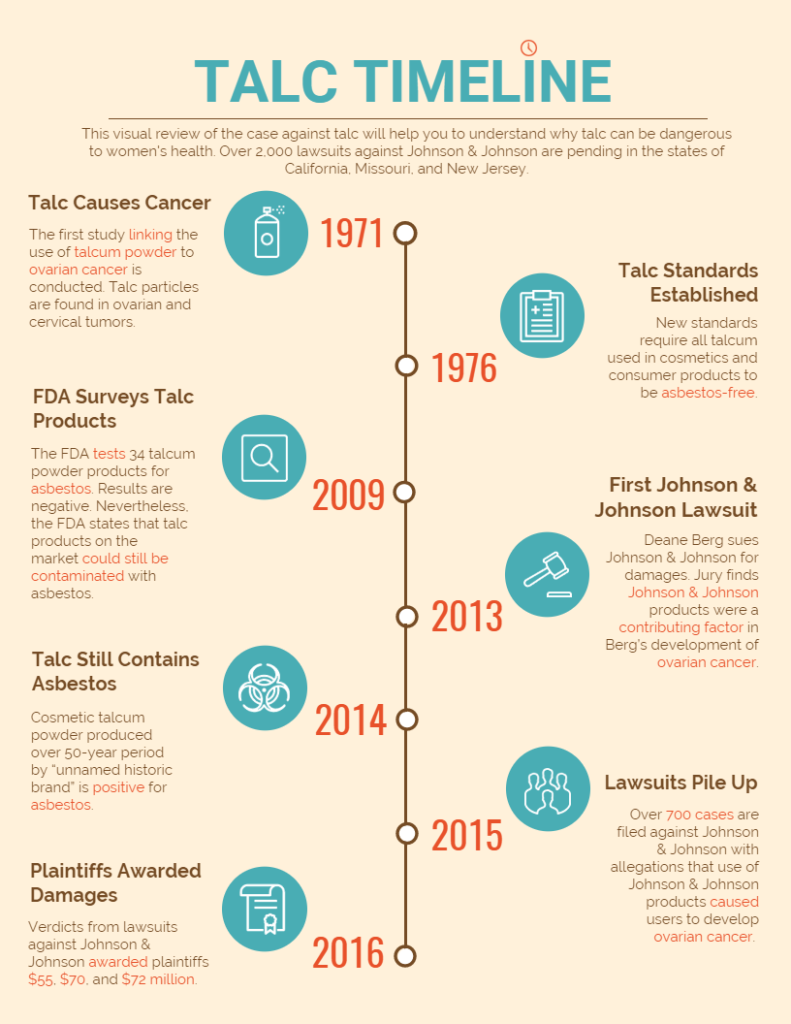
Early forms of talcum powder (more commonly known as baby or body powder) were discovered to contain the cancer-causing mineral asbestos. Asbestos can cause mesothelioma if it is inhaled, and ovarian cancer if it is used around a woman’s perineum.
In recent years, it has been widely believed that commercial talcum powder products are safe and asbestos-free. But new revelations in research, as well as the recent litigation against baby powder manufacturer Johnson & Johnson, have made it clear that cosmetic products containing talc can put women’s health at risk.
Asbestos and Talcum Powder
In 1976, after it was discovered that many talcum powder products contained asbestos, new standards were put into place requiring talc to be purified before it could be used in consumer products. Consumers were still questioning the safety of talcum powder in the early 2000s, and in 2006 the International Agency for Research on Cancer (IARC) declared that extended use of talc could be carcinogenic.
By 2010, the Food and  Drug Administration (FDA) had responded to increasing concerns by conducting a survey of 34 different cosmetic products. The products were made with talcum powder from four different talc suppliers. Some of the cosmetic powders reviewed by the FDA included:
Drug Administration (FDA) had responded to increasing concerns by conducting a survey of 34 different cosmetic products. The products were made with talcum powder from four different talc suppliers. Some of the cosmetic powders reviewed by the FDA included:
- Johnson’s Baby Powder
- CVS Brand Baby Powder
- Rite Aid Baby Powder
- Angel of Mine Baby Powder
- Shower to Shower Morning Fresh Absorbent Body Powder
At the time of the survey, the FDA did not find any evidence of asbestos in the products, but it issued a statement that the results “[did] not prove that most or all talc or talc-containing cosmetic products currently marketed in the United States are likely to be free of asbestos contamination.”
It’s worthwhile to note that the FDA does not formally regulate cosmetic products or ingredients. While the U.S. Pharmacopeial Convention (USP), a nonprofit organization that sets standards for pharmacological products, has set standardized tests for the presence of asbestos, the FDA has suggested that their standards lack specificity. The lack of clear standards and the fact that companies self-regulate their products for asbestos increases the likelihood that asbestos-contaminated products could slip through the cracks and make it to the market.
Could Commercial Talcum Powders Still Contain Asbestos?
In 2014, researchers conducted a study of products produced by an unnamed “historic” brand of cosmetic talcum powders. More than 50 samples of talcum powder produced over a 50-year period were tested in three separate laboratories. All of the samples were positive for the presence of asbestos.
While the study did not name the historic brand in question, the researchers did note that the talc which was positive for asbestos was produced in three regions: “the Willow Creek mine in Southwest Montana, the Regal mine near Murphy, North Carolina, and imported talc from the Val Chisone region of the Italian Piedmont.” The current supplier of Johnson & Johnson talcum powder has active mines in or near two of these regions — one in the Val Chisone region and another within six miles of Willow Creek, Montana.
Johnson & Johnson currently faces more than 2,000 lawsuits over allegations that its talcum powder products have caused ovarian cancer in consumers. In 2016, three plaintiffs were awarded $55 million, $70 million and $72 million for damages caused by the use of Johnson & Johnson products. If Johnson & Johnson products do, in fact, contain asbestos, it could spell a significantly more difficult battle for the cosmetic manufacturer.
How Is Talcum Powder Similar to Asbestos?
Numerous studies dating back to the 1970s have found links between the use of talcum powder and ovarian cancer. A recent meta-study published in the European Journal of Cancer Prevention found that women who used talcum powder were 20% more likely to develop ovarian cancer than women who did not use talcum powder. The study examined data of over 300,000 women from 24 previously published studies. It represents one of the largest-scale compilations of data linking ovarian cancer and talcum powder to date.
Talc is similar to asbestos both in its biological form and in the effect it has on the body. Both talc and asbestos are fibrous in their natural forms. When introduced to the body system, both minerals have also been shown to cause inflammation and suppression of the immune system. In the case of asbestos, these factors are known to play a role in the development of ovarian cancer. The fact that talc can cause a similar reaction in the human body supports the idea that talc itself, and not just talc contaminated with asbestos, could cause ovarian cancer.
Making Changes After Using Talcum Powder Products
There are many cornstarch-based alternatives to baby or body powders containing talc. Consider switching to one of these if you have been using a cosmetic talcum powder like Johnson’s Baby Powder, Baby Magic Baby Powder, or Shower to Shower. It’s also a good idea to consult your OBGYN about your talcum powder use, and make sure that you have regular screenings to ensure your ovarian and gynecological health.
Click here to view a PDF version of this infographic.