Risperdal Risperidone
A powerful anti-psychotic, Risperdal is responsible for a number of life changing complications
What is Risperdal?
Otherwise known as its generic name Risperidone, Risperdal is a powerful antipsychotic drug approved by the US Food and Drug Administration (FDA) in 1993 for the treatment of schizophrenia in adults and adolescents. It was later approved to treat bipolar disorder in adults and adolescents and autism spectrum disorders in children and adolescents.
Risperdal is used by some physicians for the treatment of ADHD, anxiety, depression, and sleep disturbances–although this is without FDA approval. Risperdal is manufactured by Janssen Pharmaceuticals Inc., a Johnson & Johnson company.
The drug has been the subject of litigation in recent years. In 2013, Johnson & Johnson agreed to pay billions in criminal and civil fines to settle accusations of improper conduct between 1999 and 2005. These accusations related to Johnson & Johnson’s improper marketing of the drug to older adults, children, and people with developmental disabilities. Patients who have taken Risperdal and developed gynecomastia, a condition which causes the abnormal enlargement of male breast tissue, have successfully sued Johnson & Johnson. The drug also carries a black box warning.
How does Risperdal work?
Risperdal is available as a tablet and in liquid form (with a dose ranging from 0.25 mg to 6mg), or as a long-acting injection (at a dose range of 12.5 to 50 mg injected by a doctor every two weeks). Doctors will base the dosage and formulation of Risperdal they prescribe on the patient’s age, medical condition, and other medications the person is taking.
Risperdal is an atypical antipsychotic drug. It is atypical because of its design to restore the balance of the naturally occurring brain chemicals serotonin and dopamine. Experts think this type of drug is more effective in reducing psychotic or aggressive behavior.
Even though it isn’t approved by the FDA, doctors regularly prescribe Risperdal for the treatment of ADHD and for aggressive and psychotic behavior in elderly patients with dementia.
SIDE EFFECTS of Risperdal
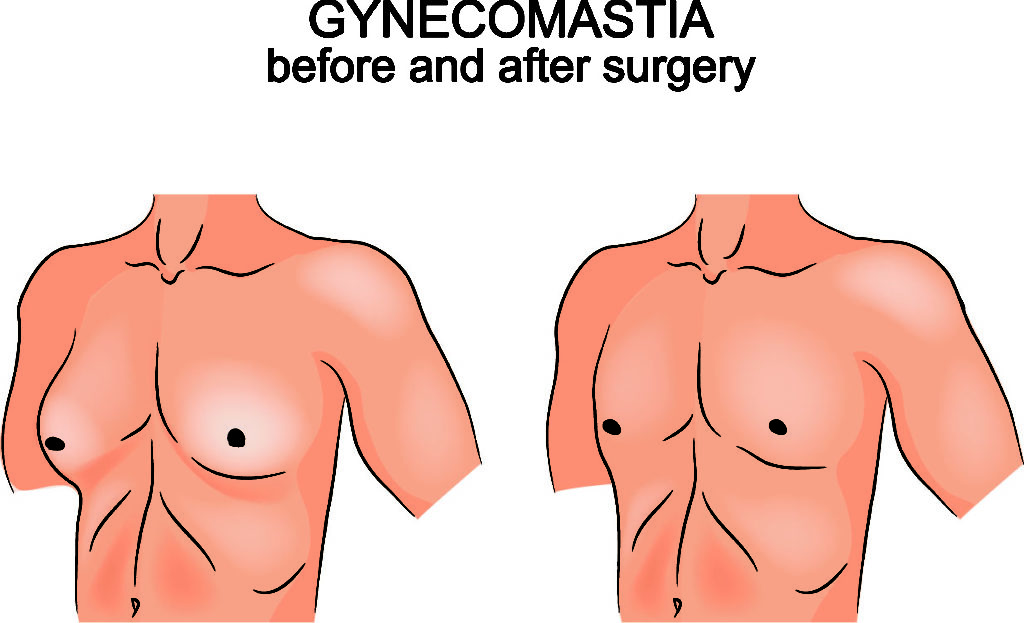
Risperdal is responsible for a large number of side effects. Of these, the one of greatest concern for many users is gynecomastia. Gynecomastia is an enlargement of breast tissue in boys and men. The condition can effect one or both breasts, either evenly or unevenly. This condition can cause chronic physical pain, but it is also a source of deep shame and embarrassment for many who are affected. Gynecomastia brought on by Risperdal use may require surgery to correct. Gynecomastia also can result in decreased sexual activity and inability to produce sperm.
There are a number of other side effects from Risperdal, which can impact many body systems including the muscular, digestive, and mental systems. The tremors and weight gain that Risperdal may cause can lead to many other health impacts, including injury and high cholesterol, respectively.
Common Side Effects
- Drowsiness
- Dizziness
- Drooling
- Weight gain
- Increased cholesterol
- Nausea
- Rise in blood sugar
Serious Side Effects
- Mental or mood changes
- Anxiety
- Restlessness
- Difficulty swallowing
- Shaking and tremors
- Muscle spasms
- Signs of infection such as fever and sore throat
- Interrupted breathing during sleep
- Gynecomastia (enlargement of male breast tissue)
Who is at Risk?

Anyone who has taken Risperdal is at some risk of side effects. The risk of side effects varies depending on age, sex, other medical conditions and medications, and for what purpose the drug has been prescribed. Men and boys, in particular, are more likely to be impacted by Risperdal complications, specifically gynecomastia.
Evidence of Risperdal Complications

In rare cases, there are a number of potentially life-changing complications of taking Risperdal. The FDA has issued a black box warning on the drug due to some of these serious health risks.
Risperdal can cause movement conditions known as tardive dyskinesia (TD) and extrapyramidal symptoms (EPS). These conditions can cause unusual/uncontrolled movements (especially of the face, lips, mouth, tongue, arms, or legs) that are sometimes painful, and in some cases are permanent.
Risperdal has also been found to increase levels of the hormone prolactin in the body. In women this may cause unwanted breast milk, missed or stopped periods, or difficulty becoming pregnant. In men, it can result in decreased sexual ability, inability to produce sperm, or enlarged breasts, a condition known as gynecomastia.
This medication may rarely cause a very serious condition called neuroleptic malignant syndrome (NMS). Symptoms of NMS include fever, severe exhaustion, severe confusion, sweating, muscle stiffness/pain/tenderness/weakness, a fast or irregular heartbeat, or signs of kidney or bladder problems (such as dark urine or changes in urination).
The black box warning was issued in relation to the serious health risks the use of Risperdal poses in treating psychosis, aggression, and behavior disorders in dementia patients. The FDA issued the warning in 2005 after it determined that the drug may lead to serious side effects or death. The warning states that there are increased mortality rates in elderly patients who are prescribed Risperdal for dementia-related psychosis, and that it is not approved for such use in patients.
It is thought that metoclopramide may interact with Risperdal.
Last Edited: February 22, 2018